Sohan Healthcare Pvt Ltd has state-of-the-art dedicated facility for manufacturing METFORMIN API. The total area for manufacturing Metformin API in Plant 1 is of 80000 sq ft.
We are having complete integration for Metformin, right from API to Finished formulation. With regulatory documentation support accompanied with EU GMP Certificate for each stage.
Upcoming API Facility: Adjoining to Plant 1, we are coming up with multi-purpose API manufacturing facility.
The Manufacturing facility is been audited and approved by all major leading health authority listed as below;
R&D plays a major role in company's strategy for creating new products and adding features to old ones. We have successfully completed developments for few molecules which are of interest for our future commercial business.
We can also undertake contract development of the API’s on exclusive basis for our clients and can also support with contract manufacturing depending of these APIs on the basis of business feasibility.
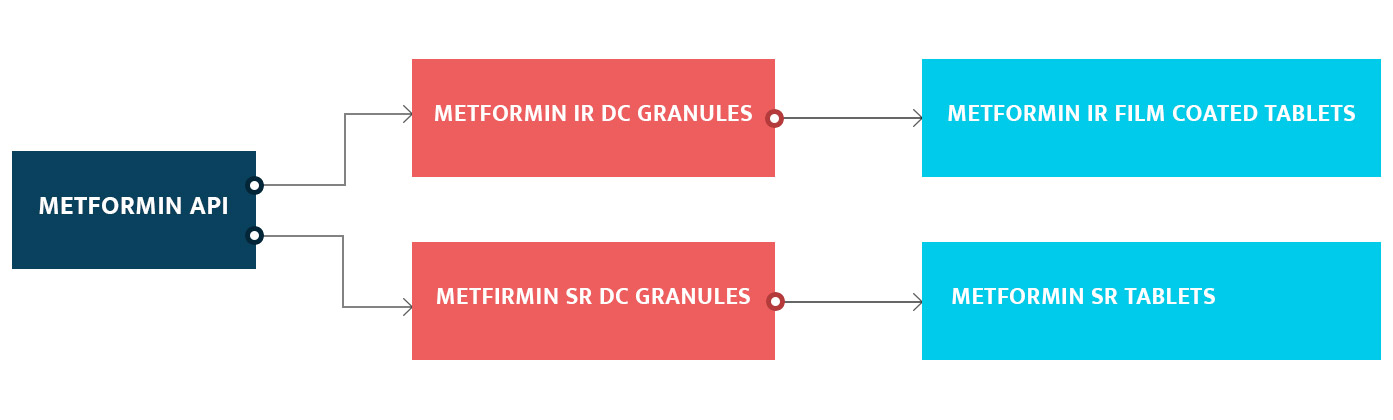
Plant has facilities / Equipments to manufacture directly compressible Granules and Pellets. Manufacturing support is from small scale to large scale production. This facility is constructed in total area of 90000 sq ft.
Directly compressible Granules: We have equipments to manufacture the various types of granules in two below listed processes.
Pellets: We have equipments to manufacture the various types of pellets with below listed techniques.
European Health Authority has audited and approved our Semi finished formulation facility.
We have a state-of-the-art pharmaceutical R&D facility for Finished Formulation as per global standards and we have a team of experienced Scientists who have experience developing different ranges of Formulations like Oral Solid Dosage, Liquids, Semi Solids and Injectables. Also, our Finished Formulation R&D works as a backbone for our semi-finished formulation plant. This facility can be used for Development as well as for small volumes of high-value products for commercial supply.